Calcium Chloride
- CAS Number: 10043-52-4
- Appearance: Solid
- Purity:%
- Made in: China
- Phone Num : +86-2150591759
- E-mail: info@shanghaimetex.com
- Description
- analysis
Description
 Tel:+86-2150591759 Tel:+86-2150591759 |
 pdf download pdf download |
 Email: info@shanghaimetex.com Email: info@shanghaimetex.com |
Calcium chloride is an ionic chemical compound that is present in anhydrous and dihydrates forms. This mineral is in the form of white crystals in standard conditions, which can well absorb moisture from the environment.
This salt is used in high purity percentages as an additive in the food industry and for food processing. This ionic compound has many similarities to table salt.

Calcium chloride is one of the most important and practical mineral chemicals that is widely used in drilling industries as drilling mud. CaCl2 is the chemical formula of this salt.
Calcium chloride is also known by other names such as calcium dichloride or chloric calcium. The main uses of this mineral are in the food industry, oil and gas industry, polymer industry, especially rubber industry. This ionic compound is also used as a coating on road surfaces and prevents the formation of ice on roadways.
The chemical structure of this ionic chemical is as follows:
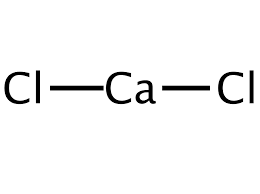
This mineral compound is generally harmless to plants and is more effective as a disinfectant at low temperatures than sodium chloride.
In recent years, the use of this ionic chemical has increased, most of which has been in demand for the oil and gas industry. In the following, we will learn more about the properties and characteristics of this chemical salt and its various applications.
Read more: Potassium sorbate
Physical and chemical properties:
Calcium chloride is relatively stable and non-flammable, easily soluble in water and glycerin, and its dissolution process releases energy and also heat and can reduce the freezing point and melting point of the solution.
This salt is one of the most abundant minerals in the earth’s crust. It is better to prevent this ionic compound from spreading in the wastewater.
The most important physical and chemical properties of this mineral can be summarized in the following table:
| Name | calcium chloride |
| Molecular weight (g/mol) | 110.98 |
| Density (g/cm³) | 2.15 |
| Melting point (°C) | 772 |
| Boiling point (°C) | 1935 |
| PH | 8 |
| Solubility in water | Completely soluble |
Synthesis and production:
The process of producing calcium chloride can be done by two different methods, which we will describe in the following two cases:
The first method is performed by the reaction of limestone and hydrochloric acid, during which carbon dioxide and calcium chloride are obtained, during this method a good concentration of this mineral can be produced in presence of a certain acid.
In some cities like Frankfurt, which have good access to limestone, this method is one of the best options for producing this salt.
The second method, which is more common in the United States, concentrates and purifies saltwater or salt deposits in lakes. Existing sediments may contain some magnesium that can be purified.
Similar product: Maltodextrin
Applications and uses of calcium chloride:
In general, calcium chloride is produced in both food and industrial grades, both of which have many applications in important industries.
- This salt is used as an antifreeze and melting point reducer for water on winter roads to prevent freezing.

- This ionic chemical is mainly used as an additive in the food industry. This salt is used in canned vegetables and packaged foods and fruits. This mineral can increase the shelf life of food products and maintain quality.
- The use of this chemical is very common in the production of beverages and cheeses. In the production of cheeses, this salt can help to form the best quality of cheeses.
- Canned tomatoes usually contain this ionic chemical, which helps to increase shelf life and improve the taste of the food.
- Calcium chloride is also used in soybean production. This mineral does not have any perceptible taste in the final product but it makes an important mineral for treating and preventing osteoporosis.

The concrete industry:
Calcium chloride is first mixed with concrete water and then added to a mixture of sand and cement so that this salt has an accelerating role in the construction of these concretes.
swimming pools and aquariums:
This chemical is used to increase the hardness of pool water. Increasing the concentration of calcium in water reduces the dissolution of the compounds required in the concrete structure and thus reduces the erosion of concrete in the pool.
Calcium chloride is used in aquariums to provide the biological calcium needed by some aquatic animals such as snails. The advantage of using this substance is that it has the least effect on the acidity of water.
Safety:
Calcium chloride in small amounts is non-toxic, but when you are working with it, all safety and health points should be observed, moist it can have inflammatory effects, avoid direct contact with eyes and skin.
Maintenance:
Choose a cool, dry place to store calcium chloride and keep packages away from heat and direct sunlight.
analysis













