Trisodium citrate
- CAS Number: 6132-04-3
- Appearance: solid powder
- Purity:%
- Made in: China
- Phone Num : +86-2150591759
- E-mail: info@shanghaimetex.com
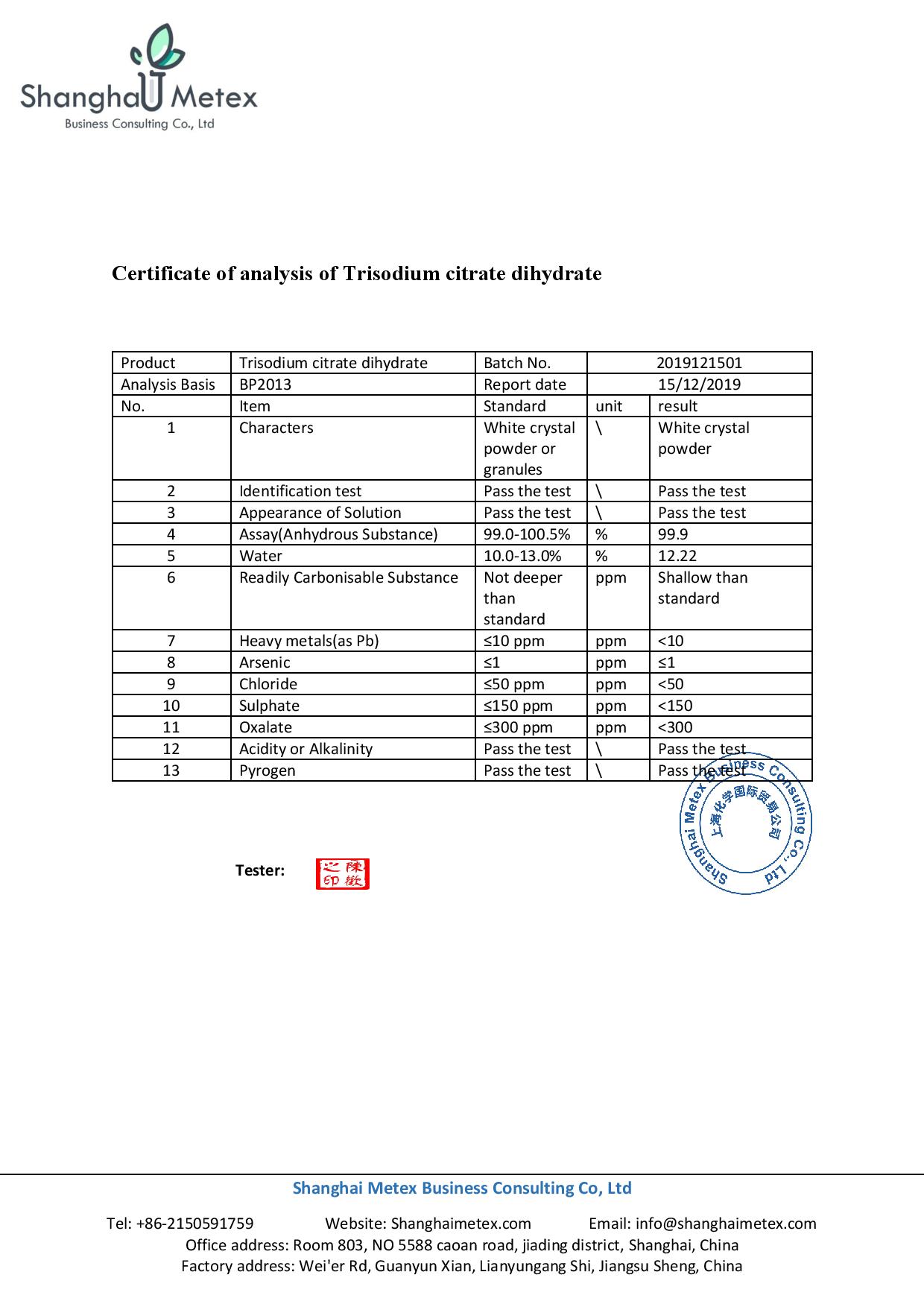
- Description
- analysis
- shipping & delivery
- Analysis
Description
Trisodium citrate with IUPAC name Trisodium 2-hydroxyproline-1,2,3-tricarboxylate is a weak base and has a sour and salty taste. There are two types of this product: anhydrous and dehydrate. By neutralization of citric acid with pure sodium hydroxide or carbonate, Trisodium citrate dihydrate is prepared. In the food industry, Na3C6H5O7 is used as preservatives and in other fields used as anticoagulants in blood and biologically buffers, and its effects in the prevention of kidney stones. For information on how to buy this product and place an order, you can contact our colleagues in the sales department.
Where to buy Trisodium citrate?
shanghai metex is ready to offer various brands of raw materials required by different industries. All you have to do is contact us through the mentioned communication channels.
Properties:
| Chemical formula
|
Na3C6H5O7 |
| Molar mass
|
258.06 g/mol (anhydrous),
294.10 g/mol (dihydrate) |
| Appearance
|
White crystalline powder |
| Density
|
1.7 g/cm3 |
| Melting point | > 300 °C (572 °F; 573 K) (hydrates lose water ca. 150 °C) |
| Boiling point | Decomposes |
| Solubility in water | Pentahydrate form: 92 g/100 g H2O (25 °C) |
| Chemical structure |  |
What is Trisodium citrate used in?
This product is also referred to as sodium citrate, has many applications in different parts. Some of them are mention below:
In food
- As an additive, usually for flavor or as a preservative. For example, use as a flavoring agent in club soda and also to contribute a tart flavor in ready-to-drink beverages.
- As a chelating agent, to prevent spoilage of foods.
- As a pH control agent, for increasing preservative.
- As an emulsifier for making cheese.

In medical
- As an anticoagulant in blood transfusions
- To relieve discomfort in urinary tract infections
- As an osmotic laxative
- As an antacid to reduce the risks associated with the aspiration of gastric contents

In industrial
- As an industrial cleaner
- To produce various goods such as inks, paints, coatings, agrochemicals, plastics, polymers, textile, and leather
- Boiler descaling
Safety information:
Research and studies show this sodium salt has no side effects on the body, so World Health Organization (WHO) is generated as safe food.
In some cases, this salt of citric acid has side effects such as muscle twitching or cramps, swelling or weight gain, weakness, mood changes, rapid and shallow breathing, fast heart rate.
According to the MSDS paper mention some points important:
- inhaled: provide fresh air.
- skin contact: rinse and then wash skin with water and soap.
- eye contact: rinse cautiously with water for several minutes.
- f ingesting: rinse the mouth.
analysis

shipping & delivery