Sodium Acetate
- CAS Number: 127-09-3
- Appearance: Solid
- Purity:%
- Made in: China
- Phone Num : +86-2150591759
- E-mail: info@shanghaimetex.com
- Description
Description
 Tel:+86-2150591759 Tel:+86-2150591759 |
 pdf downloed pdf downloed |
 Email: info@shanghaimetex.com Email: info@shanghaimetex.com |
Sodium acetate is a type of mineral salt, this chemical compound is also known by other names such as sodium ethanoate or sodium ethane.
Sodium acetate is an important substance in the food industry that its appearance in natural conditions is a colorless and odorless powder and dissolves well in water, the chemical formula of this substance is C2H3NaO2.

this natural salt is widely used in the textile industry, biotechnology, polymer industry, especially in the rubber industry, produced of processed cotton, and widely used in dialysis machines.
the chemical structure of this substance is as follows:
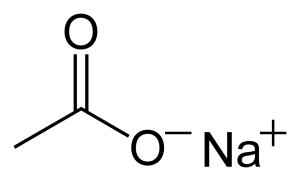
Sodium acetate can be toxic and corrosive, this mineral is prone to ignition in hot places and at high temperatures, when you are working with it, it is better to observe all safety and health points.
Sodium acetate is known as hot ice and is produced by the reaction of sodium carbonate and hydroxide.
As mentioned, the main use of this chemical is as a flavoring in food products, for example, in the production of vinegar chips, this substance is widely used.
In the following, we will become more familiar with the applications and physical and mechanical properties of Hot ice.
Read more: Aspartame
Solubility and Physico-chemical properties of Sodium Acetate:
Hot ice is easily dissolved in water, sulfur dioxide, and alcohol, this salt absorbs water well and chemically is a stable substance, this chemical is used as a preservative in cosmetic products. The use of this salt in the production of cotton clothes prevents the accumulation of static electricity.
The most important physical and chemical properties of this mineral can be summarized in the following table:
| Name | Sodium Acetate |
| Molecular weight (g/mol) | 82.0343 |
| Density (g/cm³) | 1.53 |
| Melting point (°C) | 324 |
| Boiling point (°C) | 881.4 |
| PH | 5.2 |
| Solubility in water | Completely soluble |
Synthesis and production:
The production of sodium acetate is relatively simple and can be easily produced at home or in the laboratory.
Another method of producing hot ice is the reaction between hydroxide and acetic acid.
Applications and uses of sodium acetate:
here are the most common application of sodium acetate;
Food industry:
In the production of food products, the use of sodium acetate delays the process of spoilage. This mineral salt can prevent the growth of bacteria and increase the taste quality of food. In the production of pickles, this substance can be used in abundance. Used, this adds a very salty or sweet taste to the food and greatly increases the shelf life of the product.

Laboratory applications:
Hot ice is a very useful reagent in molecular biological and biochemical laboratories, this salt can be used to extract DNA from cells.
industrial applications:
There is a relatively large amount of sulfuric acid in industrial wastewater that sodium acetate can neutralize well. With its acidic and cleansing properties, this material can help remove impurities, stains and be used as an additive in the process of producing synthetic rubber.
cosmetic products:
This substance is generally used in the production of cosmetic products as a product preservative, which can increase the quality and durability of cosmetic products.
Heating pad:
Sodium acetate is also used in heating pads and hand heaters, this solution can cool at room temperature without forming crystals. Pressing on a metal disk inside the heating pad creates a nucleation center, causing the solution to crystallize back to the solid-state of sodium hydrate trihydrate. The crystallization process is exothermic bond formation.

Sodium acetate buffer:
A mixture of weak and conjugated bases is called a buffer or buffer solution. By adding a small amount of strong acid or base to the buffer solutions, the solution becomes acid resistant, a solution of acetic acid and sodium acetate, for example. The buffer solution contains a weak acid and salt. This buffer solution is used as a tool to keep the acidity of the solution constant.
Safety:
This chemical can be chemically corrosive and toxic, avoid breathing and direct contact with the skin of your hands, the entry of this substance into the human eye can be very dangerous and severely irritate the eye.
Contact with the skin can cause inflammation and inhalation of the gas emitted from it are very harmful to the liver and throat, use gloves, goggles, and special clothing when working with it.
Transporting and storage:
Use special packaging to store this material and store it in a cool and dry place.
References:











